| Title | Pages |
|---|---|
| Preface Dear readers of the Hacettepe Journal of Biology and Chemistry, It is a great pleasure to present this year’s third issue of the Hacettepe Journal of Biology and Chemistry. This special issue includes selected publications from 15th National Chromatography Congress, which held in beautiful and historical city Uşak, on April 8-10, 2015 with the hospitality of Uşak University. Researchers, academics and students from different universities presented oral and poster presentations. The topics covered different chromatographic techniques and their applications, proteomic studies, biosensor design and applications, molecularly imprinted polymers, affinity therapy systems etc. Both oral and poster sessions were of high quality and provided a great opportunity for exchanging information and discussion on technical and scientific issues. 
|
Preface |
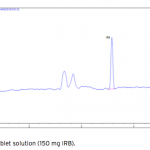
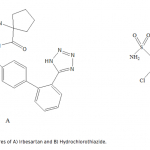
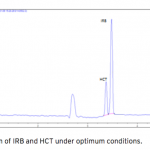
| Simultaneous Determination of Irbesartan and Hydrochlorothiazide in Tablets by CE-DAD Acapillary electrophoretic method was developed and validated for the simultaneous determination of irbesartan and hydrochlorothiazide in tablets. The run buffer was used 10 mM borate buffer containing 10% methanol (pH 9.0). Compounds were separated less than 6 minutes. The limit of quantification (LOQ) values were 3.370×10-7 M (0.144 μg/mL) and 3.140×10-7 M (0.094 μg/mL) for irbesartan and hydrochlorothiazide, respectively. The method was applied successfully to both irbesartan and irbesartan/hydrochlorothiazide combi- ned tablets with good recoveries almost 100%. 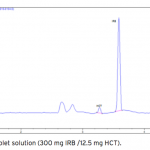
|
145 - 152 |
| Determination of Volatile Composition of Ozone Treated Arils From Longterm-Stored Whole Pomegranate Fruits Using HS- SPME GC/MS Technique Long-term stored whole ‘Hicaznar’ pomegranate fruit arils were exposured to 0 mg/h/m3, 5.2 mg/h/m3, 10.4 mg/h/m3 ozontreatmentandthenminimallyprocessedpomegranatearilsstoredduring12days.Effectsof ozone treatment to ‘Hicaznar’ pomegranate arils fruits volatile composition was analyzed by using HS-SPME/ GC/MS techniques. Ozone treated fruits showed stress volatiles such as ethanol. However acetaldehyde, acetic acid volatiles which are occured ripe fruits lower in 5.2 mg/h/m3 ozone treated pomegrante arils compared others concentrations. The identificated of arils most abundant compounds were terpenes, aldehydes, acids, alcohols and esters respectively
|
153 - 157 |
| RP-LC Determination of Dissociation Constants and Quantitative Estimation of Antiulcer drugs, Famotidine, Nizatidine and Ranitidine in their Dosage Forms Dissociation constant value (pKa) is key parameter for predicting the extent of the ionization of a drug molecule at different pH. In this study, pKa values of famotidine, ranitidine and nizatidine in different percentages of acetonitrile-water binary mixtures (10%, 15%, and 20%, v/v) were determined from the mobile phase pH dependence of retention factor with reverse phase liquid chromatographic method (RPLC). From calculated pKa values, the aqueous pKa values of studied compounds were estimated by mole fraction of acetonitrile. Moreover, the correlation established between retention factor and the pH of the water-acetonitrile mobile phase was used to determination of optimum separation condition. In order to validate the optimized condition, famotidine, ranitidine and nizatidine were studied in their dosage forms. A X-Terra C-18 reverse-phase column (250x4.6mm I.D., 5 μm particles) was preferred to carry out the developed method. Mobile phase of acetonit- rile-methanol-water (10:6:84, phosphate buffer pH: 6.5) and diode array detection system at wavelengths 210 and 320 nm were used as separation conditions. Under studied conditions detection limits of 0.5678 μg/mL for famotidine, 0.3100 μg/mL for ranitidine and 1.3144 μg/mL for nizatidine were found. The parameters, linearity, precision, accuracy, limit of detection, and limit of quantitation were studied according to U.S. Pharmacopoeia. 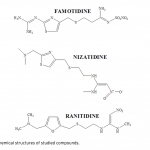

|
159 - 166 |
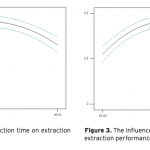
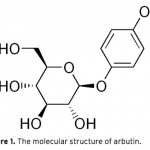
| Optimization of Ultrasound-Assisted Extraction of Arbutin from Pyrus Communis L. leaves by Response Surface Methodology Arbutin is a naturally occurring derivative of hydroquinone. It is found in various plant species belonging to diverse families, such as Lamiaceae, Ericaceae, Saxifragaceae and Rosaceae. It inhibits tyrosinase and has been employed as a cosmetic skin whitening agent. In this study, the ultrasound assisted extraction of arbutin from Pyrus Communis L. leaves was modeled using responce surface methodology. A three-level-three-factor Box–Behnken design was employed to optimize three extraction variables, including extraction temperature (X1), extraction time (X2), and methanol concentration (X3), for the achievement of high extraction yield of the arbutin. The optimized conditions are extraction temperature of 43.37oC, methanol concentration of 56.81%, extraction time of 29.66 min. Under this optimized conditions, the experimental yield of arbutin is 3.10%, which is well matched with the predicted yield of 3.12%. 
|
167 - 178 |
| Poly(hydroxyethyl methacrylate)-co-N-methacryloyl-(L)- histidine methyl ester Based Cryogel for the Removal of Fe3+ from Human Plasma effected with Beta Thalassemia In this study, Fe3+ adsorption was carried by using supermacroporous poly(2-hydroxyethyl methacrylate-N-methacryloyl-(L)-histidine methyl ester) [poly(HEMA-MAH)] cryogel. Poly(HEMA-MAH) cryogel was characterized by swelling tests and scanning electron microscopy. The influence of flow rate, pH, initial Fe3+ concentration, and Fe3+ adsorption from human plasma effected with beta thalassemia on the adsorption efficiency of the cryogel was investigated. The equilibrium swelling degree of poly(HEMA-MAH) cryogel was found to be 7.54 g H2O/g cryogel. Fe3+ adsorption capacity of poly(HEMA-MAH) cryogel from aqueous solution was estimated as 1.79 mg/g cryogel, while lower adsorption capacity was observed in human plasma (1.71 mg/g). It was also observed that Fe3+ could be repeatedly adsorbed and desorbed using poly(HEMA-MAH) cryogel without signi- ficant loss in its adsorption capacity. 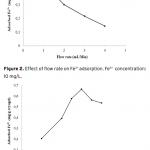
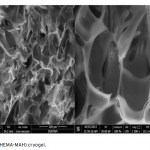
|
179 - 185 |
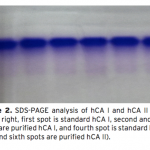
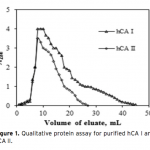
| Investigation of The Effects of Some Edible Mushroom Extracts on Human Carbonic Anhydrase Isozymes In this study the effects of four edible mushroom species (Lentinula edodes, Lactarius deliciosus, Lactarius deterrimus, Terfezia boudieri) on human carbonic anhydrase isozymes (hCA I and hCA II) were investiga- ted as in vitro. hCA I and II isozymes were purified from erythrocytes by using Sepharose®4B-L-tyrosine-p- aminobenzene sulfonamide affinity chromatography. Later the effects of the mushroom extracts on the hydra- tase and esterase activities of hCA I and II were measured. L. edodes showed inhibitory effect on the esterase activities of hCA I and II. However, other extracts showed activator effects on the esterase activities of hCA I and II. 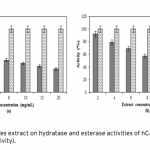
|
187 - 193 |
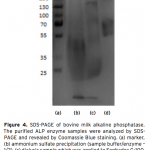
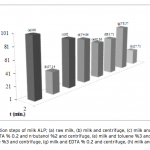
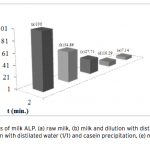
| Purification of Alkaline Phosphatase from Bovine Milk and Investigation of Inhibitory Effects Of Some Veterinary Drugs on Enzyme Activity In this study, Alkaline Phosphatase (EC 3.1.3.1, ALP) which has a critical role in phosphate metabolism was purified via bi- ochemical methods and the effects of some veterinary drugs on purified milk ALP were determined on account of the fact that using as a pasteurization indicator in milk. Enzyme was homogenized with different organic solvents, ammonium sulfate precipitation, dialysis and following Sephadex G-100 gel filtration chromatography. The enzyme product yielded bands, app- roximately 85 and 185-190 kDa on SDS-PAGE except casein fractions, while a single band, approximately 170-190 kDa interval. ALP is the most important enzyme in dairy industry as the check for the presence of residual ALP activity in milk is a good indication of proper pasteurization. Heat treatment of milk for adequate pasteurization, which kills pathogenic microorga- nisms, give rise to inactivate ALP. It seems as if milk is pasteurized before not to reach pasteurization temperature, in case of decreasing ALP activity notably by the veterinary drugs. Therefore, in vitro inhibitory effects of some drugs (furosemide, atropine sulfate, toldimfos sodium and levamisol/synonim: (-)- tetramisol HCI) were investigated in this study. While atropine sulfate didin’t effect the enzyme activity, the IC50 values of drugs which have inhibitory effect were obtained 45.48 μM, 2.19 and 126.315 mM for tetramisol, furosemide and toldimfos sodium, respectively. When compared to Levamisol, which is a po- tent inhibitor of the enzyme, the inhibitory effects of the drugs on the enzyme activity are weak. 
|
195 - 203 |
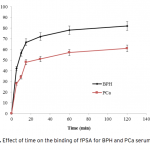
| Comparison of PSA Binding for Prostate Cancer and BPH Using Lectin-Glycoprotein Interactions The increased amount of Prostate-specific antigen (PSA) binding capacity is estimated to be associated with the initial concentration of PSA in the serum or cancer-related glycosylation patterns. The aim of this study was to determine tPSA and fPSA binding capacities of mPGMA-HDMA-Con A beads using the serum samples of patients with PCa and BPH having the same concentration of tPSA. Concanavalin A (Con A) is preferred to use as ligand due to the high sugar specificity towards mannose. The initial concentration of the Con A in the medium was changed between 0.25 and 2.5 mg/mL in order to determine the optimum amount of Con A immobilized to the beads. Both tPSA and fPSA adsorption capacity of mPGMA-HDMA-ConA beads performed using the serum sample of patient with BPH were found to be higher than that of PCa. Therefore, the reason of high PSA adsorption capacity observed in patients with BPH could be attribute to the glycosylation changes. 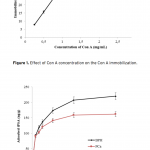
|
205 - 211 |
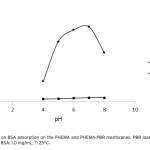
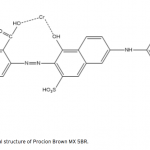
| Bovine Serum Albumin Adsorption by Dye Derived Poly(hydroxyethyl methacrylate) [PHEMA] Membranes Procion Brown MX 5BR (PBR) attached poly(hydroxyethyl methacrylate) [PHEMA] membranes were used for adsorption of albumin. The poly(hydroxyethyl methacrylate) was selected as the basic component due to it’s inertness, mechanical strength, chemical and biological stability, and biocompatibility. Different amounts of PBR was covalently attached to the poly(hydroxyethyl methacrylate) membrane to prepare poly(hydroxyethyl methacrylate)-Procion Brown [PHEMA-PBR] membrane. PHEMA-PBR membrane was characterized with Fo- urier Transform Infrared Spectroscopy (FTIR) and Scanning Electron Microscopy (SEM). Dye attached PHEMA membranes were used in bovine serum albumin (BSA) adsorption studies to assess the effects of pH, protein concentration, flow rate, temperature and ionic strength. The maximum albumin adsorption of PBR attached PHEMA membrane was 14.79 mg/g at pH 7.0 from aqueous solutions. Desorption of albumin was studied with 1.0 M NaCI solution in a continuous system. The reusability of the adsorption medium was tested for 4 ad- sorption-desorption cycles. Adsorption isotherms fitted Freundlich adsorption model and adsorption kinetics followed pseudo-second order model. 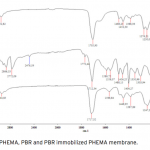
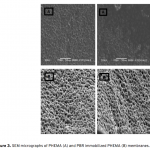
|
213 - 223 |
| Adsorption of BSA on Metal Loaded Monosize p(GMA) Microspheres Immobilized metal ion affinity chromatography (IMAC) is an efficient method for the adsorption of histidine- having proteins so poly(glycidyl methacrylate) [p(GMA)] monosize microspheres with an average diameter of 1.6 μm were produced by dispersion polymerization for adsorption of bovine serum albumin. Cu(II) ions were chelated on the poly(glycidyl methacrylate)-iminodiacetic acid [p(GMA)-IDA] microspheres to be used in bovine serum albumin adsorption studies in batch system. Characterizations of the microspheres were carried out by scanning electron microscopy (SEM), elemental analysis, Fourier transform infrared (FTIR) spectroscopy. The maximum adsorption capacity of the p(GMA)-IDA-Cu(II) microspheres was found to be 278.6 mg/g polymer at pH 5.0. The elution studies were performed by 1.0 M NaCl solution. The obtained results showed that the metal chelated p(GMA)-IDA-Cu(II) microspheres can be evaluated as an efficicent adsorbent for albumin adsorption. 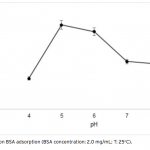
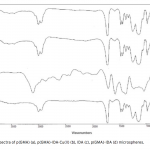
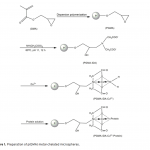
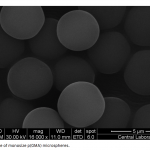
|
225 - 233 |
| Removal of Phenol and Chlorophenols from Aquatic System Using Activated Clinoptilolite Chemical contamination of water from a wide range of toxic compounds, in particular aromatic molecules, is a serio- us environmental problem owing to their potential human toxicity. In this study, activated clinoptilolite from Manisa- Gördes region (AMGC) was investigated for removal of phenol and chlorophenols (i.e. o-chlorophenol, m-chlorophenol and p-chlorophenol). AMGC was characterized by FT-IR, TG/DTA, BET Surface Area, SEM, XRD, and XRF methods. Adsorption rates of phenol and chlorophenols were very high, and equilibrium was achieved in about 45 min. The maximum adsorptions of phenol and chlorophenols onto the AMGC were 7.977 mg/g for phenol, 9.846 mg/g for o-chlorophenol, 9.981 mg/g for m-chlorophenol, and 8.241 mg/g for p-chlorophenol. The effect of various parameters like adsorbent dose, pH and initial concentration were studied for their optimization. The adsorption values of phenol and chlorophenols decreased with incre- asing pH. Desorption of phenol and chlorophenols was achieved using ethanol solution (30%, v/v). The rate of adsorption of phenol and chlorophenols were found to be maximum at pH 6.25. Equilibrium adsorption data for phenol and chlorophenols were analyzed by using Freundlich and Langmuir adsorption isotherms. It was found that Freundlich adsorption isotherm is the most suitable model. 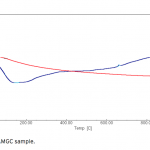

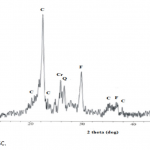

|
235 - 249 |