| Title | Pages |
|---|---|
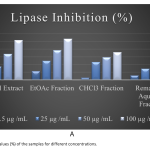
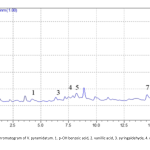
| Investigation of the Therapeutic Value of Verbascum pyramidatum Bieb. for Obesity Nowadays, one of the therapeutic approaches for obesity is the use pancreatic lipase inhibitors which reduce the digestion and absorption of fats. Most research indicates that natural sources which have a lipase inhibitory effect, may be utilized to treat obesity. Verbascum pyramidatum is one of the potential natural sources for obesity, and it has been demonstrated to have anti-inflammatory, anti-diabetic, and regulatory effects on lipid metabolism. With this study, V. pyramidatum's potential lipase inhibitor effect, it is aimed to reveal its value in the treatment of obesity. In vitro spectroscopic method was used to determine the lipase inhibitory effect of V. pyramidatum. The quantitative investigation of V. pyramidatum's phenolic metabolites with anti-obesity activity was carried out utilizing the Reverse Phase-High Performance Liquid Chromatography method. In this reported study, it was proven that extract and all fractions had an impact that inhibited lipase, with the ethyl acetate extract showing the highest inhibitory effect. Additionally, it was revealed through HPLC analysis that the species included p-OH benzoic acid, coumaric acid, quercetin, sinapic acid, and syringaldehyde. It has been demonstrated that V. pyramidatum may be a promising candidate for obesity treatment, but further investigations are required to use it as a therapeutic agent. 
|
251 - 258 |
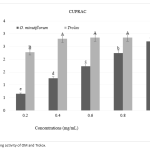
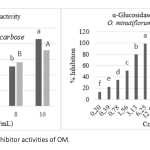
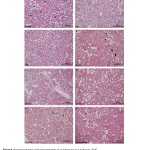
| Antidiabetic and Antioxidant Effect of Origanum minutiflorum O. Schwarz & P. H. Davis in Streptozotocin-Induced Diabetic Rats In this study, the antidiabetic potential of the aqueous extract of Origanum minutiflorum was tested using streptozotocin induced diabetic rats. Changes in body weight, blood glucose level, biochemical parameters, antioxidant enzyme activities and histopathological examination of kidney were evaluated. The administration of the aqueous extract and carvacrol to diabetic group rats caused a significant decrease in blood glucose, biochemical parameters of serum and significantly increased the concentration of high density lipoprotein cholesterol. The administration of the aqueous extract and carvacrol significantly increased the antioxidant enzyme activity and reduced the MDA level. The histopathological analysis of kidney showed that the aqueous extract and carvacrol had a possible ameliorative effect against kidney damage. The aqueous extract also showed α-amylase and α-glucosidase inhibitor activity with high antioxidant activity. These scientific results confirm the use of O. minutiflorum as adjuvant antidiabetic therapy. 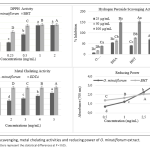
|
259 - 270 |
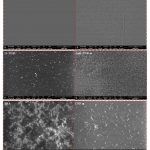
| 11-(Triethoxysilyl) Undecanal Agent-Based Biosensor System Using Disposable ITO- PET Electrode for Tumour Necrosis Factor-Alpha Detection In this study, a label-free electrochemical biosensor system based on a disposable indium tin oxide polyethylene terephthalate (ITO-PET) electrode modified with the 11-(triethoxysilyl) undecanal (11-TESU) agent was developed for the detection of tumour necrosis factor-alpha (TNF-α) in serum. The developed biosensor was observed with electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) techniques, square wave voltammetry (SWV) and single frequency impedance (SFI) technique which is utilized for the specific interaction between anti-TNF-α and TNF-α antigen. In addition, scanning electron microscopy was used to look at how the morphology of each ITO-PET surface changed (SEM). All parameters such as 11-TESU concentration, anti-TNF-α concentration and anti-TNF-α incubation time, were optimized. The biosensor system was characterized by measuring its linear determination range, repeatability, reproducibility, reusability, storage stability, and surface coverage. The TNF-α electrochemical biosensor showed high levels of repeatability and reproducibility as well as a large dynamic range of detection (from 0.03 pg mL-1 to 3 pg mL-1). The LOD and LOQ for the biosensor were extremely low at 1x10-4 pg mL-1 and 5x10-4 pg mL-1, respectively. It was applied to real samples to determine whether the proposed biosensor would be useful in clinical settings. 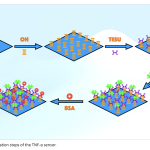


|
271 - 281 |
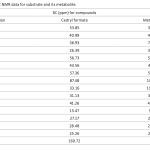
| Fungal Biotransformation of Cedryl Formate Cedryl formate is a synthetic fragrance compound, which is an ester derivative of the sesquiterpenoid cedrol, found in different parts of the cedar tree/cedarwood with a characteristic fragrance. Cedryl formate is used in cosmetics and personal care products like fragrances, perfumes, as well as in the chemical and agriculture industries in various forms. In the present work, the microbial transformation of the formate was evaluated by 13 fungal cultures to produce new derivatives. Among the evaluated, the plant pathogenic fungus Aspergillus niger NRRL 326, biotransformed to the metabolite 8-cedren-3β-ol derivative with 6.2% yield. The structure of the chromatographically purified metabolite was elucidated by NMR and spectroscopic methods. 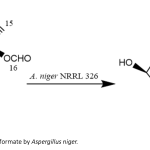
|
283 - 287 |
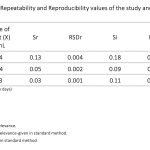
| A Standard Method Verification for Determination of Sugar Content of Commercial Fruit Juices by HPLC This study aimed to verify the standard test method TS EN 12630 and determine the sugar contents of commercially available fruit juices by high pressure liquid chromatography. A laboratory should verify the standard test method parameters in order to show its performance for the analysis, under consideration. For this purpose, Sucrose, Glucose and Fructose were analysed in orange juices obtained from the market. The principle of the method is based on the separation of sugars on a cation-exchange resin by isocratic elution with mobile phase, detection using a differential refractive index (RI) detector and external standard method. Accuracy and precision were performed via intraday and inter day studies to determine of accuracy and the precision (generally accepted as repeatability and reproducibility) for the standard test method. The recovery values of the sugars added into juice sample were found 92%, 99% and 96% for sucrose, glucose and fructose, respectively. 
|
289 - 295 |
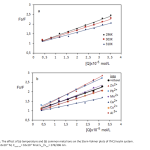
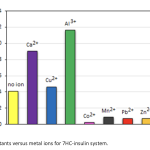
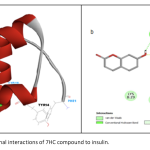
| Investigation on the Binding properties of a Coumarin Derivative to Insulin by Spectroscopic and Computational Approaches The binding properties of insulin hormone to the potential antidiabetic coumarin derivative umbelliferone (7hydroxycoumarin, 7HC) was investigated by absorption, fluorescence quenching and molecular docking methods. The negative signs of thermodynamic parameters (ΔH and ΔS) indicated that hydrogen bonds and van der Waals forces were dominant in the binding mode. The effect of common metal ions was investigated on binding parameters. According to the Förster’s theory; binding distance, r was obtained as 4.17 nm. The spectral data further supported by molecular docking calculations which show hydrogen bonds between 7HC and insulin. 
|
297 - 307 |
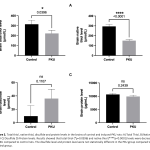
| Thiol/Disulfide Balance in Induced Phenylketonuria Model Objective The rare metabolic disorder phenylketonuria (PKU) is caused by a deficiency in the phenylalanine hydroxylase enzyme. Deficiency of this enzyme causes phenylalanine accumulation in the brain and irreversible neurological damage by increasing the blood phenylalanine level. The purpose of this study was to determine how the phenylketonuria model affected the balance of thiols and disulfides in the brain. Method: Brain total thiol and native thiol levels were measured by the modified elman method in rat pups (n:7) in which PKU model was generated and control group (n:7). The disulfide level was calculated according to the total thiol and native thiol levels. Results: The brain total thiol level of the PKU group was statistically decreased compared to the control group (*p=0.0369). Brain native thiol level of the PKU group was statistically decreased compared to the control group (****p<0.001). The brain disulfide level of the PKU group did not differ statistically compared to the control group (p=0.1107). Conclusion: It was concluded that oxidative stress in PKU may affect thiol/disulfide levels. The study, which was reported for the first time in the literature, showed a change in the thiol/disulfide balance in the PKU model. 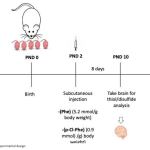
|
309 - 315 |
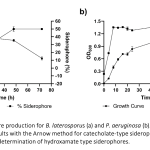
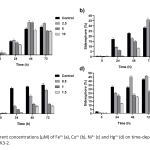
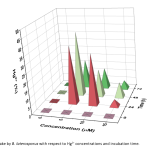
| Investigation of the Siderophore Production and Associated Heavy Metal Accumulation Potential of Brevibacillus laterosporus 301/İK3-2 Siderophores are secondary metabolites released into the environment by various microorganisms, fungi and plants to chelate iron from the surrounding environment. It is known that siderophores bind to other metals besides iron. Today, heavy metals, which are released as an undesirable result of industrial development, accumulate at high rates and pose a significant threat to biological living things. In this sense, remediation of heavy metal contaminated sites is an urgent requirement. Siderophores are promising agents for the removal of heavy metals from natural habitats with the role of bioremediation. In this study, the effect of heavy metals on the growth and siderophore production of Brevibacillus laterosporus was investigated. Maximum siderophore production was determined as 50 % at 48 h in the metal free growth media. In addition, maximum siderophore production was determined in the presence of various heavy metals including 5 μM Hg2+, 0.5 mM Ni2+, 0.1 mM Co2+ and 2.5 μM Fe2+. Intracellular uptake of the mercury was also measured using optical emission spectroscopy and compared with siderophore production values of the B. laterosporus. The maximum biosorption of mercury was measured to be 40% in 5 μM Hg2+ containing media at 48 h of incubation. The results show that siderophore production is affected by uptake of various metals, and are usable for removing of heavy metals from environmental habitats. 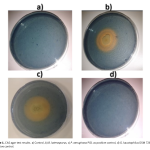
|
317 - 325 |