Special Issue for 2nd International Environmental Chemistry Congress
Edited by Bekir Salih
Last update 15 January 2021
Chemistry is being increasingly applied to aid in data mining and to improve results interpretability for environmental problems. Environmental problems could be resulted from not only chemistry but also biological processes. But, again chemistry and also biology should be responsible to solve the problems in the environment. That’s why, the subject nicely fits within the scope of Hacettepe Journal of Biology and Chemistry, which is devoted to all aspects and phases of biological and chemical analysis, covering fundamental aspects, instrumentation, new developments, innovative ideas, new sample preparation techniques for different physical state samples such as air, liquid and solid, and applications all these in the environmental area.
To show the recent advancements in the area, we introduce a special issue entitled “Environmental Chemistry”. Reports describing new or improved analytical protocols, as well as the modification/automation of instruments, aimed at monitoring the presence of all type of contaminants (inorganic and organic) in different samples. This special issue covers the solutions of some environmental problems providing important methodological developments of relevance to addressing questions using analytical chemistry and biological techniques in environmental area. All reviews and research papers followed a rigorous, peer-review process. This special issue covered the articles those were presented 2nd International Environmental Chemistry Congress taken place Antalya-TURKEY on 31st October-3rd November 2019.
| Title | Pages |
|---|---|
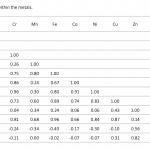
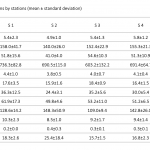
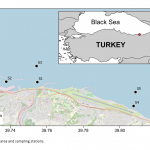
| Determination of Metal Concentrations in Değirmendere Stream Drainage Area of Southeastern Black Sea Coast of Turkey This study aims to determine the metal concentrations and enrichment levels in the drainage area of the Değirmendere Stream located Southeastern Black Sea coast of Turkey. Sediment samples were collected with Ekman grab from five stations in three periods. Aluminum (Al), Vanadium (V), Chrome (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu), Zinc (Zn), Arsenic (As), Cadmium (Cd) and Lead (Pb) concentrations in the sediment samples were measured with ICP-MS after digestion with a microwave system. Differences in metal concentrations between the stations were not statistically significant (p>0.05). However, the concentration of many metals (Fe, Mn, Co, Ni, Cu, Zn, V) in station 1 at the river mouth was relatively higher than the others. The enrichment factor (EF) by normalized with aluminium were 1.5-2.1 for V; 0.6-1.0 for Cr; 0.8-1.7 for Mn; 1.1-1.7 for Fe; 1.1-2.0 for Co; 0.4-0.9 for Ni; 1.3-2,3 for Cu; 1.4-3.9 for Zn; 0.8-1.4 for As; 0.5-4.1 for Cd and 0.9-4.2 for Pb. Moderate enrichments were observed for Cu Zn, Pb in the some stations. 
|
117 - 124 |
| Modern Methods for Wastewater Treatment The article considers the photochemical degradation of the phenol solution in the presence of TiO2 nanoparticles, which synthesized in moderate conditions and dimensions vary between 10-30 nm and the 100 mg/ml NH4OH solution. The appearance of photochemical dissociation was confirmed on the basis of the absorption curves taken before and after the process and Abs curves were drawn on the “Varian” device. Phenol’s photochemical dissociation in UV-visible area has been confirmed by experiments. 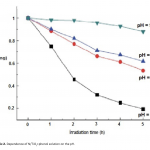
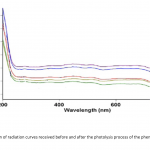
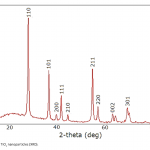
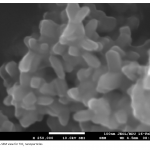
|
125 - 131 |
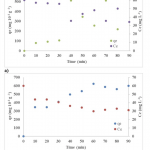
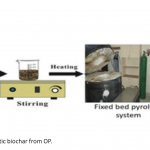
| Phenol Adsorption on Magnetic Biochar Derived From Olive Pomace: Equilibrium, Kinetic and Thermodynamics In this study, magnetic biochar obtained from pyrolysis of pretreated olive pomace by iron chloride was used as adsorbent to remove phenol and the adsorption capacity of phenol was revealed. Batch experiments were performed as a function of pH, contact time, adsorbent dosage, temperature, and phenol concentration. Moreover, adsorption kinetics and thermodynamics of phenol adsorption onto magnetic biochar were also evaluated in the study. The optimum conditions for maximum adsorption capacity were obtained at pH of 5.7, dosage of biochar 0.14 g and 60 minutes contact time. In this study, three adsorption isotherms, namely Langmuir, Freundlich and Temkin, were applied to fit the equilibrium data of adsorption of phenol onto magnetic biochar. Results showed that correlation coefficients (R2) for three isotherm models decreased with the temperature increment from 20°C to 40°C and the most suitable isotherm model for adsorption was Freundlich. As for kinetics of the adsorption process, the best described model was found as pseudo-second order. In adsorption thermodynamics part, the negative ΔH° and ΔG° values demonstrated that adsorption was exothermic, feasible and was more spontaneous at lower temperatures. 
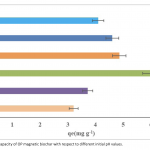
|
133 - 145 |
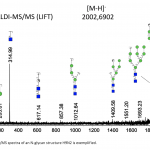
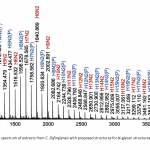
| N-Glycosylation Profiles of the Green Microalgae Chlorella Zofingiensis Nowadays, the use of microalgae species as raw materials in biopharmaceutical production is on the agenda. The reason behind this idea is that microalgae are cell factories that are able to efficiently utilize carbon dioxide for the production of numerous biologically active compounds. However, there are several problems that remain to be solved in the production of recombinant protein from microalgaes. One of the critical requirements is to produce a bio-compatible N-glycosylation profile from the secreted recombinant proteins. The knowledge about the glycosylation machinery and N-glycan profiles of microalgae spices are quite limited. In the study, it was aimed to characterize N-glycan profiles of a green microalgae, Chlorella zofingiensis. To achieve this, photoautotrophically grown Chlorella zofingiensis extracts including (glyco-)proteins were enzymatically deglycosylated and labelled with 2-aminobenzoic acid tag. Released N-glycans were purified with a HILIC-based approach and analyzed by MALDI-TOF(/TOF)-MS. The results showed that C. zofingiensis included oligomannosidic type N-glycan patterns. In addition, N-glycosylation profiles of C. zofingiensis by MALDI-MS revealed that most of the oligomannosidic N-glycans were phosphorylated. 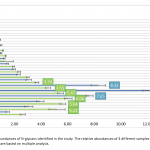

|
147 - 155 |
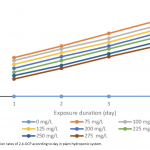
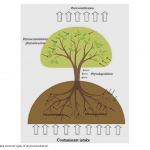
| Investigating Usage Potential of Datura stramonium L. for Phytoremediation of 2,4-Dichlorophenol In this work, the phytoremediation potential of 2,4-Dichlorophenol (2,4-DCP) from soil and wetlands by Datura stramonium L. (jimsonweed) was investigated. The medium of seedlings growing in a hydroponic system was adjusted to different concentrations (0.0, 75, 100, 125, 150, 175, 200, 225, 250 and 275 ppm) of 2,4-DCP. Four days later, the remediation rate of 2,4-DCP in the growth medium, and root-stem length, root-stem dry weight, lipid peroxidation (LPO), protein and photosynthetic pigment content of seedlings were evaluated. D. stramonium seedlings provided remediation of 2,4-DCP between 52-78% at all concentrations. In addition, the 2,4-DCP treatments inhibited the root-stem lengths and dry weights of seedlings compared to their controls, particularly at high doses such as 200-275 ppm, but not at low doses. The applications generally increased protein and LPO content of roots and leaves slightly, but did not affect chlorophyll. The results show that D. stramonium has a high usage potential for phytoremediation of 2,4-DCP. 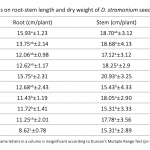

|
157 - 166 |
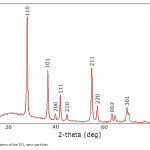
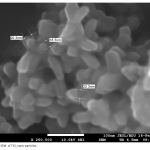
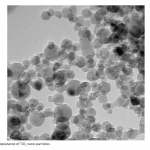
| Photodegradation of Phenol Using N/TiO2 System In the article the photochemical dissociation of phenol in the participation of TiO2 nano-particles and methyl-3-aminocrotonate was done for the first time, the period was taken as 60 minutes, the processing of photochemical dissociation was verified by the curves drawn for reaction solution in the UV radiation device and the 60% decomposition of phenol was defined. Light absorption of a system with TiO2 is observed only in the UV area, whereas absorption of a system with N/TiO2 falls on the visible area of the spectrum. 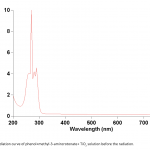
|
167 - 173 |
| Charge Recombination Suppressed CdSeS/CdSe/ZnS QDSSC Design The interest in quantum dot sensitized solar cells (QDSSC), which has theoretically proved to have up to 44% energy conversion efficiency in recent years, is growing rapidly. Although it has theoretically high efficiency value, PCE obtained in studies with QDSSCs is far from these values. This situation shows that there are many difficulties to be solved in QDSSC technology. One of the main challenges in QDSSC technology is irradiated load recombination occurring in QDSSC. For this reason, in this study, it is about using CdSeS QDs as an alternative to the most used CdS QDs in the literature in order to suppress the load recombination between TiO2 surface and electrolyte and QD surfaces. In the study, while CdS and CdSeS QDs were coated on the TiO2 surface with SILAR method, the previously synthesized CdSe QD was coated with chemical deep deposition method. Surfaces were last treated with ZnS QDs. An optimization study was carried out to determine the ideal number of CdSeS coatings for QDSSCs. As a result, the Jsc and Voc values for TiO2/CdSeS4/CdSe/ZnS QDSSCs were 8.799 mA/ cm2 and 0.795 V, respectively, while the PCE value increased to 4.452%. 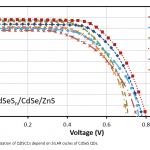


|
175 - 184 |